Difference between revisions of "Corona Detective"
| Line 52: | Line 52: | ||
When the pandemic began, RA (as CSO of AGiR!) of course began some corona virus 'sequence gazing' ... | When the pandemic began, RA (as CSO of AGiR!) of course began some corona virus 'sequence gazing' ... | ||
Thomas Landrain's visit to Hackuarium was put off by 'lockdown' - but a big collaboration developed in the platform of his Just One Giant Lab (JOGL). | Thomas Landrain's visit to Hackuarium was put off by 'lockdown' - but a big collaboration developed in the platform of his Just One Giant Lab (JOGL). | ||
| − | RA got Guy and Fran in too, and one thing led to another... Here is our [https://www.youtube.com/watch?v=Tdfws3g3R2s 'teaser' video] from when we were semi-finalists for the xprize! | + | RA got Guy and Fran in too, and one thing led to [https://app.jogl.io/project/181 another]... Here is our [https://www.youtube.com/watch?v=Tdfws3g3R2s 'teaser' video] from when we were semi-finalists for the xprize! |
The first 'workshop' of this [https://app.jogl.io/project/181/coronadetective method to detect the virus] causing Covid-19, inspired by the [https://gmodetective.com/ GMO Detective], was held on the evening of 9June21, and was virtual, as part of the [https://bbkopenscience.com/streaming/ BBK open science festival]!<br> | The first 'workshop' of this [https://app.jogl.io/project/181/coronadetective method to detect the virus] causing Covid-19, inspired by the [https://gmodetective.com/ GMO Detective], was held on the evening of 9June21, and was virtual, as part of the [https://bbkopenscience.com/streaming/ BBK open science festival]!<br> | ||
| Line 72: | Line 72: | ||
*the whole JOGL 'project nucleic amplification team' and Thomas Landrain for support! | *the whole JOGL 'project nucleic amplification team' and Thomas Landrain for support! | ||
*you? | *you? | ||
| − | |||
===Main Goals === | ===Main Goals === | ||
| Line 83: | Line 82: | ||
==Recommendation + next steps == | ==Recommendation + next steps == | ||
| − | + | (The following is from January 2022) Organising reliable GMP batches for independent validation by regulators has been a great difficulty, with it being hard to reach agreements at such a distance with no actual meetings. Additionally, it seems likely that licensing agreements are needed, as not only did extension of LAMP patent get approved, but there is a patent for the QUASR detection method depicted above (from the Sandia national labs in the states...). | |
Currently, we are in discussion with people in Ghana as those moronic variants get spread... (yes, sorry, sometimes only humor can help us realise - if people already knew more when this started and were strict the first 2 months, we might not be here 2 years later! -almost, yes...) | Currently, we are in discussion with people in Ghana as those moronic variants get spread... (yes, sorry, sometimes only humor can help us realise - if people already knew more when this started and were strict the first 2 months, we might not be here 2 years later! -almost, yes...) | ||
| + | |||
| + | |||
| + | Now, more than a year later (Feb 2023), we are learning to live with Covid-19, but our PoP batch of reactions from Canada's freeze-drying company EVIK, has still not finished providing amazing data. Here in Switzerland, clinical validations with left over RNA extracts were about 97% concordant for positive and 100% for negative samples. We await results from our colleagues in Ghana, and also 96 reactions are in Chile, for further tests versus batches made with 'homebrew' enzyme batches and clinical tests (perhaps also with colleagues in Brazil). The compiled results may help for the next pandemic and make open science innovation more acceptable in its early phases? | ||
| + | |||
| + | |||
| + | ''(Ce qui suit date de janvier 2022) L'organisation de lots BPF fiables pour une validation indépendante par les régulateurs a été une grande difficulté, car il est difficile de parvenir à des accords à une telle distance sans réunions réelles. En outre, il semble probable que des accords de licence soient nécessaires, car non seulement l'extension du brevet LAMP a été approuvée, mais il existe également un brevet pour la méthode de détection QUASR décrite ci-dessus (des laboratoires nationaux Sandia aux États-Unis...).'' | ||
| + | |||
| + | ''Actuellement, nous sommes en discussion avec des personnes au Ghana alors que ces variantes débiles se répandent... (oui, désolé, parfois seul l'humour peut nous aider à réaliser - si les gens en savaient déjà plus quand cela a commencé et étaient stricts les 2 premiers mois, nous ne serions peut-être pas là 2 ans plus tard ! -presque, oui...)'' | ||
| + | |||
| + | ''Aujourd'hui, plus d'un an plus tard (février 2023), nous apprenons à vivre avec Covid-19, mais notre lot de réactions PoP de la société canadienne de lyophilisation EVIK, n'a toujours pas fini de fournir des données étonnantes. Ici en Suisse, les validations cliniques avec les extraits d'ARN restants étaient concordantes à 97 % pour les échantillons positifs et à 100 % pour les échantillons négatifs. Nous attendons les résultats de nos collègues au Ghana, et aussi 96 réactions au Chili, pour des tests supplémentaires par rapport aux lots fabriqués avec des lots d'enzymes "maison" et des tests cliniques (peut-être aussi avec des collègues au Brésil). Les résultats compilés pourraient être utiles pour la prochaine pandémie et rendre l'innovation scientifique ouverte plus acceptable dans ses premières phases ?'' | ||
[[Category:Work In Progress]] | [[Category:Work In Progress]] | ||
[[Category:Manual]] | [[Category:Manual]] | ||
Revision as of 13:56, 12 February 2023
(française dessous)
This last pandemic year has brought together a lot of open collaborative research, including...
The Corona Detective Project
Here is our recent simple one page summary to seek more partners to help get this out where it is needed most!
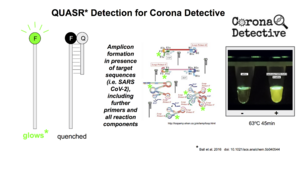
We have a new graphic, QuasrGraphic3 (below right), to explain how the amazing 'quenched fluorescence' detection method works, which provides such clear positive and negative endpoint results in Corona Detective reactions.
Because we have focussed on open science and securing GMP 'at scale' production, and not made a company directly, people are not sure how to deal with us, perhaps to the project's detriment. One international colleague first asked to license our open protocols, and most recently was asking for our recommendations for a lyophiliser. =)
(this colleague is helping fill the 1h test result market for 200 bucks a pop... )
Possibly we should have just gone to the Swiss regulators early on, with reaction tubes freeze-dried at CRI.
XPRIZE, outside of getting us more control results (other viruses, too) was basically only fun...
but again this is because our 'business model' is inexistant. LOL
maybe you could help?
Le lien au dessus va vers notre récent et simple résumé d'une page pour rechercher d'autres partenaires afin d'aider à diffuser ce produit là où il est le plus nécessaire !
Nous avons créé un nouveau graphique, QuasrGraphic3 (en bas à droite), pour expliquer le fonctionnement de l'étonnante méthode de détection par "fluorescence éteinte", qui fournit des résultats positifs et négatifs si clairs dans les réactions du Détective de Corona.
Parce que nous nous sommes concentrés sur la science ouverte et sur l'assurance d'une production GMP "à l'échelle", et que nous n'avons pas créé directement une entreprise, les gens ne savent pas comment traiter avec nous, peut-être au détriment du projet.
Par exemple, un collègue international a d'abord demandé une licence pour nos protocoles ouverts et, plus récemment, il nous a demandé nos recommandations pour un lyophilisateur. =)
(ce collègue contribue à remplir le marché des résultats de tests en une heure pour 200 dollars la pièce...)
Nous aurions peut-être dû, dès le début, nous en remettre nos réactifs aux régulateurs suisses, avec des tubes de réaction lyophilisés au CRI.
XPRIZE, en dehors du fait qu'il nous a permis d'obtenir plus de résultats de contrôle (d'autres virus aussi), n'était fondamentalement qu'un amusement...
mais encore une fois, c'est parce que notre "modèle économique" est inexistant. LOL
Vous pourriez peut-être nous aider ?
Introduction
This wiki page is under construction, to provide more information about this method for surveillance testing!
Context
When the pandemic began, RA (as CSO of AGiR!) of course began some corona virus 'sequence gazing' ... Thomas Landrain's visit to Hackuarium was put off by 'lockdown' - but a big collaboration developed in the platform of his Just One Giant Lab (JOGL). RA got Guy and Fran in too, and one thing led to another... Here is our 'teaser' video from when we were semi-finalists for the xprize!
The first 'workshop' of this method to detect the virus causing Covid-19, inspired by the GMO Detective, was held on the evening of 9June21, and was virtual, as part of the BBK open science festival!
We had 4 labs (here, in Paris, in Bilbao, and in Zurich) running tests that day.
Happily, all 4 cities had great positive control results and no people positive... whew. (hope for video stream link ...)
The latest 'simple' protocol is available here, and your input is welcome.
Versions in French and Spanish are available too, already!
With our Colombian colleagues we also discussed the method virtually also, as the 2nd of a 3-part series of talks (Oct2020, as announced in the flyer, Charla #2).
People
Contact and lead:
- Rachel Aronoff - Founder and CSO of AGiR! Action for Genomic integrity through Research!
Participants:
- Tatiana Eliseeva
- Guy Aidelberg
- Fran Quero
- the whole JOGL 'project nucleic amplification team' and Thomas Landrain for support!
- you?
Main Goals
Surveillance screening for Covid-19
Objectives
- open science collaboration to develop a sensitive molecular diagnostic method
- testing and validation
- manufacturing at scale, to obtain regulator approval for surveillance screening (also of those with no symptoms)
Recommendation + next steps
(The following is from January 2022) Organising reliable GMP batches for independent validation by regulators has been a great difficulty, with it being hard to reach agreements at such a distance with no actual meetings. Additionally, it seems likely that licensing agreements are needed, as not only did extension of LAMP patent get approved, but there is a patent for the QUASR detection method depicted above (from the Sandia national labs in the states...).
Currently, we are in discussion with people in Ghana as those moronic variants get spread... (yes, sorry, sometimes only humor can help us realise - if people already knew more when this started and were strict the first 2 months, we might not be here 2 years later! -almost, yes...)
Now, more than a year later (Feb 2023), we are learning to live with Covid-19, but our PoP batch of reactions from Canada's freeze-drying company EVIK, has still not finished providing amazing data. Here in Switzerland, clinical validations with left over RNA extracts were about 97% concordant for positive and 100% for negative samples. We await results from our colleagues in Ghana, and also 96 reactions are in Chile, for further tests versus batches made with 'homebrew' enzyme batches and clinical tests (perhaps also with colleagues in Brazil). The compiled results may help for the next pandemic and make open science innovation more acceptable in its early phases?
(Ce qui suit date de janvier 2022) L'organisation de lots BPF fiables pour une validation indépendante par les régulateurs a été une grande difficulté, car il est difficile de parvenir à des accords à une telle distance sans réunions réelles. En outre, il semble probable que des accords de licence soient nécessaires, car non seulement l'extension du brevet LAMP a été approuvée, mais il existe également un brevet pour la méthode de détection QUASR décrite ci-dessus (des laboratoires nationaux Sandia aux États-Unis...).
Actuellement, nous sommes en discussion avec des personnes au Ghana alors que ces variantes débiles se répandent... (oui, désolé, parfois seul l'humour peut nous aider à réaliser - si les gens en savaient déjà plus quand cela a commencé et étaient stricts les 2 premiers mois, nous ne serions peut-être pas là 2 ans plus tard ! -presque, oui...)
Aujourd'hui, plus d'un an plus tard (février 2023), nous apprenons à vivre avec Covid-19, mais notre lot de réactions PoP de la société canadienne de lyophilisation EVIK, n'a toujours pas fini de fournir des données étonnantes. Ici en Suisse, les validations cliniques avec les extraits d'ARN restants étaient concordantes à 97 % pour les échantillons positifs et à 100 % pour les échantillons négatifs. Nous attendons les résultats de nos collègues au Ghana, et aussi 96 réactions au Chili, pour des tests supplémentaires par rapport aux lots fabriqués avec des lots d'enzymes "maison" et des tests cliniques (peut-être aussi avec des collègues au Brésil). Les résultats compilés pourraient être utiles pour la prochaine pandémie et rendre l'innovation scientifique ouverte plus acceptable dans ses premières phases ?