Difference between revisions of "QualityControl4Genes"
| Line 9: | Line 9: | ||
Interested to learn more? | Interested to learn more? | ||
| − | Here is the link to the recent paper | + | Here is the [https://pubmed.ncbi.nlm.nih.gov/38437914/ link to the recent paper one of the the human orthologues] of this nématode gene, ''smg-4''. |
As Nietzsche said - you have made your way from worm to man, but much within you is still worm... | As Nietzsche said - you have made your way from worm to man, but much within you is still worm... | ||
| − | This key pathway has the responsibility of removing | + | This key pathway has the responsibility of removing mRNAs that might express dominant negative or toxic fragments of otherwise good genes. Errors are normal, and all the stages of gene expression - from transcription, to splicing, to translatin to protein or activating other functions - can be subject to mistakes. Prevention is what mRNA surveillance is about. However, these genes also have developmental roles and natural targets which they may regulate. |
| − | Here is part of [https://doi.org/10.7554/eLife.33292 an open access paper]'s introduction more focused on another quality control mechanism - but it all starts with mRNA surveillance ( | + | Additional feedback loops can affect activity or even expression levels, for appropriate function. |
| + | |||
| + | Here is part of [https://doi.org/10.7554/eLife.33292 an open access paper]'s introduction more focused on another quality control mechanism - but it all starts with mRNA surveillance (which from yeast studies had been also termed 'nonsense-mediated decay'): | ||
<small>Nonsense-mediated decay (NMD) (reviewed in [He and Jacobson, 2015]) is a translational surveillance pathway to mitigate deleterious products of premature stop codons. In NMD, recognition of an early stop codon destabilizes an mRNA (Morse and Yanofsky, 1969; Baserga and Benz, 1988; Losson and Lacroute, 1979). Foundational studies in ''S. cerevisiae'' and ''C. elegans'' revealed protein factors responsible for NMD (Leeds et al., 1991; Hodgkin et al., 1989; Pulak and Anderson, 1993). In the decades since, a large body of literature has highlighted similarities and differences in NMD between yeast and metazoans. For example, while both yeast and metazoan NMD involve a core set of three proteins (UPF1-3 in yeast, SMG-2–4 in metazoans), metazoans require additional proteins for NMD (e.g. SMG-1, –5, and −6). Additionally, ''Saccharomyces cerevisiae'' NMD is thought to occur predominantly through decapping and 5’>3’ exonucleolytic degradation (Muhlrad and Parker, 1994), while studies across metazoans have implicated both exo- and endonucleolytic machineries (e.g. [Lykke-Andersen, 2002; Lejeune et al., 2003; Gatfield and Izaurralde, 2004; Glavan et al., 2006; Huntzinger et al., 2008; Eberle et al., 2009; Lykke-Andersen et al., 2014; Schmidt et al., 2015; Ottens et al., 2017]).</small> | <small>Nonsense-mediated decay (NMD) (reviewed in [He and Jacobson, 2015]) is a translational surveillance pathway to mitigate deleterious products of premature stop codons. In NMD, recognition of an early stop codon destabilizes an mRNA (Morse and Yanofsky, 1969; Baserga and Benz, 1988; Losson and Lacroute, 1979). Foundational studies in ''S. cerevisiae'' and ''C. elegans'' revealed protein factors responsible for NMD (Leeds et al., 1991; Hodgkin et al., 1989; Pulak and Anderson, 1993). In the decades since, a large body of literature has highlighted similarities and differences in NMD between yeast and metazoans. For example, while both yeast and metazoan NMD involve a core set of three proteins (UPF1-3 in yeast, SMG-2–4 in metazoans), metazoans require additional proteins for NMD (e.g. SMG-1, –5, and −6). Additionally, ''Saccharomyces cerevisiae'' NMD is thought to occur predominantly through decapping and 5’>3’ exonucleolytic degradation (Muhlrad and Parker, 1994), while studies across metazoans have implicated both exo- and endonucleolytic machineries (e.g. [Lykke-Andersen, 2002; Lejeune et al., 2003; Gatfield and Izaurralde, 2004; Glavan et al., 2006; Huntzinger et al., 2008; Eberle et al., 2009; Lykke-Andersen et al., 2014; Schmidt et al., 2015; Ottens et al., 2017]).</small> | ||
| Line 34: | Line 36: | ||
We have already confirmed old results here, with 'rescue' by PCR products, as shown in the image above right... | We have already confirmed old results here, with 'rescue' by PCR products, as shown in the image above right... | ||
| − | (To note: 'rescue' makes the worms paralysed in this genetic context.) | + | (To note: 'rescue' makes the worms paralysed in this genetic context. It is just due to the fact that a myosin heavy chain gene is the target...) |
| − | + | The study began with pcr fragment tests and attempts to KnockDown the gene with RNA. However, in fact, the RNAi feeding, even in a super-sensitive strain, did not give any effect, although the amplified transgenes worked a charm, with products from mutant strains did not rescue, and had the sequences predicted, of course. We also confirmed the deletion sequence of a mutant made with psoralen-uv, and this is interesting as it keeps the key domain, but the 900bp lost only partially rescue (egg laying, but not muscle function). | |
Injecting the premade RNP with guide RNAs and the repair template was super efficient! (Of 25 initial transformants, 5 gave the perfect edit.) | Injecting the premade RNP with guide RNAs and the repair template was super efficient! (Of 25 initial transformants, 5 gave the perfect edit.) | ||
| − | The Knockout animals have Pvuls and act as a simple loss of function, so we hypothesize the other genes in the operon have some essential function that makes it difficult to make new mutations in the region. | + | The KO was already made by March 2023 ! |
| + | |||
| + | The Knockout animals have Pvuls and act as a simple loss of function, so we hypothesize the other genes in the operon have some essential function that makes it difficult to make new mutations in the region. One allele with a complex rearrangement is less fit than others, and is likely to affect another gene in its operon. More molecular details can be seen in this preliminary slidedeck. | ||
| − | Sequence confirmation on an old deletion in the gene (but much after the original paper) gives us clues about what is most important for function | + | Sequence confirmation on an old deletion in the gene (but much after the original paper) gives us clues about what is most important for function. We speculated some essential function, partly because mutations in the gene were rare (and the only point mutations found were on either end of the same intron...). A partial loss of function from a deletion made in Japan was also confirmed during this recent work. |
In humans, there are two copies on different chromosomes, with the X-linked one tied to Intellectual Disabilities, and [https://www.cmmc-uni-koeln.de/de/research/research-areas-projects/research-area-c/niels-gehring-c-07 more interesting features] in knockdown and attempts to knock this QC mechanism out in cells... | In humans, there are two copies on different chromosomes, with the X-linked one tied to Intellectual Disabilities, and [https://www.cmmc-uni-koeln.de/de/research/research-areas-projects/research-area-c/niels-gehring-c-07 more interesting features] in knockdown and attempts to knock this QC mechanism out in cells... | ||
Revision as of 18:37, 8 March 2024
Even gene expression needs quality control!
With colleagues in Basel and the approval of of the Federal authorities in Bern, we are looking further into some molecular mysteries around all this...
Our first transgenic animals (well, as an invertebrate some say we should just call them GMO) are in the lab, and we plan to learn a lot by making some more!
Interested to learn more?
Here is the link to the recent paper one of the the human orthologues of this nématode gene, smg-4.
As Nietzsche said - you have made your way from worm to man, but much within you is still worm...
This key pathway has the responsibility of removing mRNAs that might express dominant negative or toxic fragments of otherwise good genes. Errors are normal, and all the stages of gene expression - from transcription, to splicing, to translatin to protein or activating other functions - can be subject to mistakes. Prevention is what mRNA surveillance is about. However, these genes also have developmental roles and natural targets which they may regulate.
Additional feedback loops can affect activity or even expression levels, for appropriate function.
Here is part of an open access paper's introduction more focused on another quality control mechanism - but it all starts with mRNA surveillance (which from yeast studies had been also termed 'nonsense-mediated decay'):
Nonsense-mediated decay (NMD) (reviewed in [He and Jacobson, 2015]) is a translational surveillance pathway to mitigate deleterious products of premature stop codons. In NMD, recognition of an early stop codon destabilizes an mRNA (Morse and Yanofsky, 1969; Baserga and Benz, 1988; Losson and Lacroute, 1979). Foundational studies in S. cerevisiae and C. elegans revealed protein factors responsible for NMD (Leeds et al., 1991; Hodgkin et al., 1989; Pulak and Anderson, 1993). In the decades since, a large body of literature has highlighted similarities and differences in NMD between yeast and metazoans. For example, while both yeast and metazoan NMD involve a core set of three proteins (UPF1-3 in yeast, SMG-2–4 in metazoans), metazoans require additional proteins for NMD (e.g. SMG-1, –5, and −6). Additionally, Saccharomyces cerevisiae NMD is thought to occur predominantly through decapping and 5’>3’ exonucleolytic degradation (Muhlrad and Parker, 1994), while studies across metazoans have implicated both exo- and endonucleolytic machineries (e.g. [Lykke-Andersen, 2002; Lejeune et al., 2003; Gatfield and Izaurralde, 2004; Glavan et al., 2006; Huntzinger et al., 2008; Eberle et al., 2009; Lykke-Andersen et al., 2014; Schmidt et al., 2015; Ottens et al., 2017]).
Although protective under many circumstances, the NMD pathway also contributes to pathological suppression of expression from numerous disease-causing mutations (about 11% of point mutations responsible for human disease [Mort et al., 2008]).
(Selected References from the paper excerpt, at bottom...)
Have questions?
Would you be interested to try some worm research?
Both a Crispr knock-out and knock-in (mCherry) and RNAi were planned for this molecular genetic project in the model system, C. elegans, in collaboration with the Mango lab at the Biozentrum of the University of Basel.
Here is the original paper about the cloned worm gene (Aronoff et al, 2001), about which we are learning more.
We have already confirmed old results here, with 'rescue' by PCR products, as shown in the image above right...
(To note: 'rescue' makes the worms paralysed in this genetic context. It is just due to the fact that a myosin heavy chain gene is the target...)
The study began with pcr fragment tests and attempts to KnockDown the gene with RNA. However, in fact, the RNAi feeding, even in a super-sensitive strain, did not give any effect, although the amplified transgenes worked a charm, with products from mutant strains did not rescue, and had the sequences predicted, of course. We also confirmed the deletion sequence of a mutant made with psoralen-uv, and this is interesting as it keeps the key domain, but the 900bp lost only partially rescue (egg laying, but not muscle function).
Injecting the premade RNP with guide RNAs and the repair template was super efficient! (Of 25 initial transformants, 5 gave the perfect edit.)
The KO was already made by March 2023 !
The Knockout animals have Pvuls and act as a simple loss of function, so we hypothesize the other genes in the operon have some essential function that makes it difficult to make new mutations in the region. One allele with a complex rearrangement is less fit than others, and is likely to affect another gene in its operon. More molecular details can be seen in this preliminary slidedeck.
Sequence confirmation on an old deletion in the gene (but much after the original paper) gives us clues about what is most important for function. We speculated some essential function, partly because mutations in the gene were rare (and the only point mutations found were on either end of the same intron...). A partial loss of function from a deletion made in Japan was also confirmed during this recent work.
In humans, there are two copies on different chromosomes, with the X-linked one tied to Intellectual Disabilities, and more interesting features in knockdown and attempts to knock this QC mechanism out in cells...
Update March 2024
As mentioned above, the Knockout was successful on the first attempt, with 5 independent lines obtained from 25 initial 'roller' transformantss. Crispr is amazing!
The KnockIn (to put the red mCherry marker, into the similar region as GFP was put in, for the original work 2 decades ago) was more of a challenge. Results with the KnockIn are just starting to come in, and outcrosses of the strain and crosses to put it in the context of a smg-suppressible myosin heavy chain mutation, unc-54(r293) have begun.
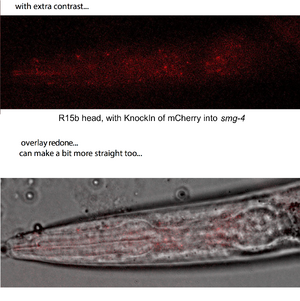
Here is one of the first epi-fluor microscopy results (thanks to our friends at the UNIL):
speckles of the Smg-4 shuttling protein in a larval brain!
More experiments to come and writing of an article! Hoping to learn much more in combination with other GFP tagged components of the pathway and nuclear (nuclear pore?) markers. If you would like to learn more too, or have ideas - do not hesitate to write! =)
contact rachel (at) hackuarium (dot) ch...
=)
References:
He and Jacobson, 2015: Nonsense-Mediated mRNA Decay: Degradation of Defective Transcripts Is Only Part of the Story. https://doi.org/10.1146/annurev-genet-112414-054639
Morse and Yanofsky, 1969: Polarity and the Degradation of mRNA. https://doi.org/10.1038/224329a0
Baserga and Benz, 1988: Nonsense mutations in the human beta-globin gene affect mRNA metabolism. https://doi.org/10.1073/pnas.85.7.2056
Losson and Lacroute, 1979: Interference of nonsense mutations with eukaryotic messenger RNA stability. https://doi.org/10.1073/pnas.76.10.5134
Leeds et al, 1991: The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. doi:
10.1101/gad.5.12a.2303
Hodgkin et al, 1989: A new kind of informational suppression in the nematode Caenorhabditis elegans. doi: 10.1093/genetics/123.2.301
...
Mort et al, 2008: A meta-analysis of nonsense mutations causing human genetic disease. https://doi.org/10.1002/humu.20763