OpenInsulin
The Open Insulin project is part of an international collaboration.
At Hackuarium, the first results are just beginning:
We are happy to have sequence confirmation of two OpenInsulin plasmids from Alex Kelly
Winsulin and ProInsulin
to produce and test (+ controls)
The project has been under discussion for quite some time, and we hope more people will want to join in for this work.
test
part1
Our goals
- Pave the way for more OpenInsulin tests with International Colleagues
- Demystify and explain the best practices toward any open science project
- Produce Winsulin and ProInsulin for open access positive controls
Why participatory research?
the means to confront anti-science sentiments is to pull more people in
learning through hands on experiences is crucial...
Why Open Data?
By making all the data generated by the project open, we want to explore and promote an alternative to proprietary initiatives. Open data supports literacy and fosters innovation by both citizens and scholars. Open designs can still be produced for commercial ventures, and can make profits if there is a market for the product.
What are the specific challenges of the project and has anyone done this before?
Open Insulin history
Hackuarium's Open Insulin Entanglements
Alex Kelly to Geneva
Plasmids finally to Hackuarium, around August 2020, for Winsulin and ProInsulin, both constructs based on periplasmic expression vectors.
Current status
Clones grown in DH5alphas and finally sequence confirmation has come through.
Complications from low copy number vector and GC-rich stretches overcome via midipreps and 'power read upgrades' for the sequencing.
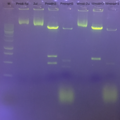
Looks great...
gel with HindIII diagnostic result.
and mini vs midipreps
seq [traces] are clean for (lengths)
Next plans
- plasmids to be put in BL21 (production E. coli strain)
- induced with IPTG to express the protein
- Cells expressing Winsulin/ProInsulin will be harvested by centrifugation and lysed using a French Pressure cell. The lysate will be centrifuged at 30'000g for 35min to remove cell debris and insoluble proteins.
- The soluble proteins will be applied to a 5ml column containing Ni-NTA resin. The resin will be washed extensively before the protein elution with a high-imidazole containing buffer
- Winsulin purified using the His-tag will be proteolytically cleaved using Tobacco Etch Virus (TEV) protease. That cleavage will remove the His-tag from the peptide.
(Oscar Vadas of unige: initial proposal)