|
|
| (16 intermediate revisions by 3 users not shown) |
| Line 18: |
Line 18: |
| | = Who = | | = Who = |
| | <br> | | <br> |
| | + | [[User: Vlorenzo | Vanessa Lorenzo]] |
| | + | Hackuarium team that helped to push this project forward: Sachiko Kana, Michael Pereira, Octanis Team, Luc Henry, Yann Pierson, Gianpaolo Rando, Yann Heurtaux, Carmelo Bisognano (Univercité), Clément Drévo and Gavrilo Bozovic. |
| | + | Xavier Perret UNIGE that provided proof of concept experiment with the Violacein at his lab. |
| | + | Naiyi Wang that designed infographics |
| | + | Alexandra Midal and Mathieu Bassée HEAD Genève that promoted the early project in 2015. |
| | <br> | | <br> |
| | | | |
| Line 24: |
Line 29: |
| | Pr(ink)t plastic, it's fantastic! | | Pr(ink)t plastic, it's fantastic! |
| | | | |
| − | Le challenge : construire un objet vivant, plein de bactéries que nous devons nourrir et soigner pour obtenir des couleurs riches pour les utiliser dans une imprimante 3D.
| + | STRAINS: |
| | + | J. Lividum |
| | + | M. Luteus |
| | + | M. Roseus |
| | + | M. Aurantica |
| | | | |
| − | Symbiose et ailleurs. Serait-il très intéressant si est possible d'interagir dans ce système biomécanique avec tes propes bactéries. Expanded self sounds good to me ;)
| + | CULTURES: |
| | + | LB medium (liquid, no agar) |
| | + | See LB protocol [http://wiki.hackuarium.ch/w/BioP_Cultures here] |
| | | | |
| − | Le projet sera présenté à l'exposition del Mobile de Milano en avril 2015.
| + | STERILISATION: |
| − | | + | Autoclave: |
| − | Certaines bactéries naturelles et non pathogènes, comme le Janthinobacterium Lividum, font des pigments violet foncé. Un mélange de produits chimiques fournira différentes couleurs (c'est aussi possible de faire papier avec levure et acide acétique).
| |
| − | | |
| − | Il faudrait :
| |
| − | | |
| − | 1.- Acheter les bactéries
| |
| − | | |
| − | 2.- Modifier une imprimante 2D
| |
| − | | |
| − | 3.- Système de transmission de bio-encre et un objet esthétique qui permettra de l'utiliser.
| |
| − | | |
| − | A la fin cela ressemblera a un petit laboratoire de bactéries sympathiques, si on a de la chance.
| |
| − | | |
| − | 07/11/2014
| |
| − | -------------------------------------
| |
| − | Learn with Clément Drévo how to make a Control Petri-dish for tracking the growth of a contaminated one with my own finger bacteria.
| |
| − | | |
| − | 12/11/2014
| |
| − | ------------------------------------
| |
| − | Learn with Gavrilo Bozovic the use of the UV bacteria "killer" and make tests with different exposure times 10' 30' 45' 60'
| |
| − | | |
| − | 19/11/2014
| |
| − | --------------------------------------
| |
| − | Buy CANON Pixma ip2850 (bubble Ink) http://www.pctop.ch/pInfos.aspx?CODE=F27A01&PRINT=TRUE
| |
| − | Found that not refillable cartridges are available but "refilable ink kit".
| |
| − | Info and instructions for how to inject ink directly in the originals of CANON Pixma ip2850 and others http://www.uni-kit.com/pdf/inkjetrefillinstructions.pdf
| |
| − | | |
| − | Check the results of the use of the UV bacteria "killer" in diferent petri dishes for different exposure times:
| |
| − | | |
| − | | |
| − | (foto from Hackuarium)
| |
| − | | |
| − | 26/11/2014
| |
| − | ----------------------------------------
| |
| − | Shopping list
| |
| − | https://docs.google.com/spreadsheets/d/1_gf--PE-xaBDfA-HcX4ACqOqOhKtS6nCOiAvjEFMHMY/edit?usp=sharing
| |
| − | | |
| − | Select with Yann Pierson a bacteria which is actuallly producing Yellow color. And preparing together with S.H. the LB medium and learn how to sterealize it in the heat"pressure cooker".
| |
| − | | |
| − | 27/11/2014
| |
| − | ----------------------------------
| |
| − | All these strains produce pigments:
| |
| − | | |
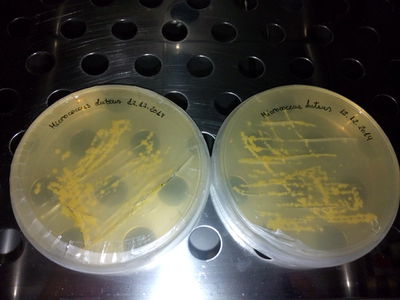
| − | Micrococcus luteus (ML DSMZ) from DSMZ color: yellow
| |
| − | Rhodococcus coprophilus (RC) from DSMZ color: pink
| |
| − | Janthinobacterium lividum (JL) from DSMZ color: blue (interesting link http://schaechter.asmblog.org/schaechter/2009/04/by-jenna-tabor-godwin-rhona-stuart-rosa-i-león-zayas-and-chitra-rajakuberan--------janthinobacterium-cultured-on-nb-aga.html)
| |
| − | Xanthomonas campestris (XC) from DSMZ color: yellow
| |
| − | Rhizobium etli (RE) from DSMZ color: brown
| |
| − | Vogesella indoferra (VI) from Dr. Simon Park color: dark blue
| |
| − | Arthrobacter agilis (Aag) from Dr. Simon Park color: pink
| |
| − | Chromobacterium violaceum (CV) from Dr. Simon Park color: purple
| |
| − | Dermacoccus nishinomyaensis (DN) from Dr. Simon Park color: orange
| |
| − | Micrococcus roseus (MR) from Dr. Simon Park color: salmon
| |
| − | Arthobacter polychromogens (AG) from Dr. Simon Park color: dark blue
| |
| − | Micrococcus luteus (ML) from Dr. Simon Park color: yellow
| |
| − | | |
| − | The purple pigment is produced by two of them:
| |
| − | - Chromobacterium violaceum
| |
| − | - Janthinobacterium lividum
| |
| − | | |
| − | Both produce Violacein. Do mind that C. violaceum is a level 2 organism, opportunistic pathogen. So. I guess the best option is the Janthinobacterium lividum.
| |
| − | | |
| − | --------------------------------------
| |
| − | | |
| − | Searching for a way to extract the pigment from the plates and for a growing method in liquid nutrition.
| |
| − | | |
| − | 03/12/2014
| |
| − | --------------------------------------
| |
| − | | |
| − | Inspiring projects:
| |
| − | http://www.ingridnijhoff.nl
| |
| − | http://www.thibault.io
| |
| − | | |
| − | (link to video from Thibault Brevet https://www.youtube.com/watch?v=p4P4RagpMLs )
| |
| − | | |
| − | 05/12/2014
| |
| − | --------------------------------------
| |
| − | Yellow chromobacteria: Micrococcus luteus (ML DSMZ) from DSMZ color: yellow, is now safe at home ;) thanks to Clément Drévo
| |
| − | | |
| − | (foto from: http://microbio146.blogspot.ch/2011_08_01_archive.html)
| |
| − | | |
| − | --------------------------------------
| |
| − | 07/12/2014
| |
| − | | |
| − | Ideal goal: bio ink printer, product design of a new ecological printer based on Continuous Ink Systems. Trying to get an unexpensive one (Epson R200, Epsonr340)
| |
| − | | |
| − | About Continuous Ink System: http://en.wikipedia.org/wiki/Continuous_ink_system
| |
| − | | |
| − | About Microbiology and Choromobacteria: http://microbio146.blogspot.ch/2011_08_01_archive.html
| |
| − | | |
| − | DIY projects with CIS: http://www.diyphotography.net/diy_continuous_ink_systems/
| |
| − | | |
| − | DIY proyects 2D printer with Arduino:
| |
| − | http://www.instructables.com/id/Homemade-arduino-printer/?ALLSTEPS
| |
| − | print characters at an XY position on LCD
| |
| − | http://playground.arduino.cc/Code/PCD8544
| |
| − | to draw and print you own glyph:
| |
| − | http://www.carlos-rodrigues.com/projects/pcd8544/
| |
| − | print controller:
| |
| − | http://www.instructables.com/files/orig/F2A/4VX1/GJQEKZ1T/F2A4VX1GJQEKZ1T.tmp
| |
| − | | |
| − | 08/12/2014
| |
| − | -------------------------------
| |
| − | Ordered Janthinobacterium Lividum. Collaboration with Prof. Xavier Perret at UNIGE.
| |
| − | | |
| − | 10/12/2014
| |
| − | --------------------------------
| |
| − | We have started to grow our little Yellow Babies! Thanks for the support Clement.
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | | |
| − | | |
| − | 11/12/2014
| |
| − | -------------------------
| |
| − | Babies are not growing in Lb medium, I try with 80 rpm and 22°C
| |
| − | I would try 37°C 200rmp (Where do bacteria grow the best ? In our bodies. What is the temperature there ? 37°C héhé)
| |
| − | | |
| − | | |
| − | | |
| − | 12/12/2014
| |
| − | -------------------------
| |
| − | This morning we discussed with Gavrilo some of the problems I faced with the process, I follow the advice of growing up more Micrococcus Luteus colonies in 2 more petri dishes.
| |
| − | | |
| − | | |
| − | False alarm, babies growing up! (I think something is going on in the erlenmeyer because of the opacity of the liquid)
| |
| − | Anyway I follow the wise advice and rised the temperature up to 37°
| |
| − | --- Vive la Suisse Tropical
| |
| − | | |
| − | | |
| − | 15/12/2014
| |
| − | ------------------------------
| |
| − | Yellow babies are growing but not in the way I want: its all the same soup of Lb medium and dizzy diving bacteria and "foam".
| |
| − | | |
| − | Have to think about a better way of making them grow: shaking speed, more food, less T°, add more density to the liquid medium?
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | On the other hand, my new two colonies are healthy pushing forward in 2 petri dishesh.
| |
| − | | |
| − | | |
| − | 16/12/2014
| |
| − | -----------------------------------------
| |
| − | M.Luteus (yellow babies) extractions started. Thanks Luc for all the help. I used this protocol http://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC212584&blobtype=pdf
| |
| − | /************************************************************
| |
| − | PLAN to start new colonies in LB medium (liquid, no agar). --> I prepare for autoclave LB medium in 5 erlenmeyer bottles (250ml):
| |
| − | 1 normal, 1 normal + 2g.Yest and 2g.tryptone, 3 normal + 4g.Yest + 4g.tryptone.
| |
| − | | |
| − | Autoclave instructions: | |
| | 0.-Plug | | 0.-Plug |
| | 1.-fill the bottom with water till reach the 1st level/grid on the bottom. | | 1.-fill the bottom with water till reach the 1st level/grid on the bottom. |
| Line 190: |
Line 51: |
| | 8.-Unplug | | 8.-Unplug |
| | | | |
| − | Finally extract the culture and empty a little quantity on the new erlenmeyer
| + | |
| − | *****************************************************************/
| |
| | EXTRACTIONS: | | EXTRACTIONS: |
| | First take some culture from the original bottle, "source". Prepare the table: | | First take some culture from the original bottle, "source". Prepare the table: |
| Line 199: |
Line 59: |
| | empty the source bottle on the new bottle | | empty the source bottle on the new bottle |
| | heat it up a bit with fire the mouth of the bottle and the aluminium foil before putting in again the aluminium foil. | | heat it up a bit with fire the mouth of the bottle and the aluminium foil before putting in again the aluminium foil. |
| − |
| |
| | | | |
| | Then we continue the process with this tools: | | Then we continue the process with this tools: |
| | + | 1.- Pipette, always use the plastic cone(aeroject) on them: We extract some an put them in tubes (then close them). |
| | + | 2.- Then we shake the tubes in the automatic vortex at 10 x 1000 and 10 minutes |
| | + | 3.- Empty the lb medium that should remain on the top, we mix the rest (the pigment), with alcohol, we put the tubes in the machine of ultrasounds, in order to break the molecules to free the pigment. (ultra strong detergent would be helpful) the tube content should seem creamy and colorful. |
| | + | 4.- and then we put them again into the vortex in order to compact the pigment and keep it as a first sample to work with, THE FIRST YELLOW INK!!! |
| | | | |
| | + | **** Notes on J. Lividum*********** |
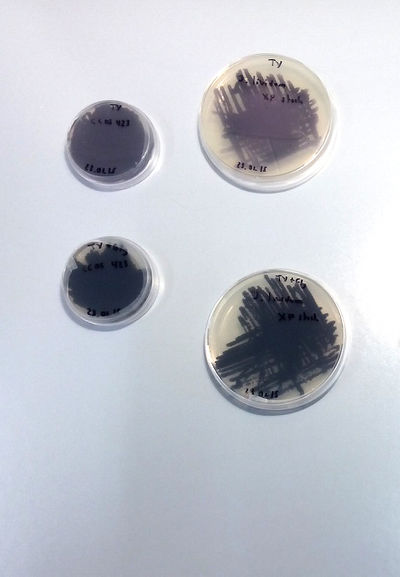
| | + | X.Perret, Senior Lecturer Microbiology Unit Laboratory of Microbial Genetics Sciences III – University of Geneva has accepted to collaborate in the project. We are working in the lab at UM under his supervision and the assistance of his students and Phd students. First Extraction following the article Pantanella-F_2007: [http://www.ncbi.nlm.nih.gov/pubmed/17381742] |
| | | | |
| − | always use the plastic cone(aeroject) on them:
| + | Culture on TY and TY + Glycerol |
| − | | |
| − | We extract some an put them in tubes
| |
| − | | |
| − | Then we shake the tubes in the automatic vortex
| |
| − | | |
| − | | |
| − | at 10 x 1000 and 10 minutes
| |
| − | | |
| − | | |
| − | Them we empty the lb medium that should remain on the top
| |
| − | we mix the rest (the pigment), with alcohol.
| |
| − | we put the tubes in the machine of ultrasounds, in order to break the molecules to free the pigment.
| |
| − | | |
| − | | |
| − | the tube content should seem like this
| |
| − | | |
| − | | |
| − | | |
| − | and then we put them again into the vortex in order to compact the pigment and keep it as a first sample to work with, THE FIRST YELLOW INK!!!
| |
| − | | |
| − | | |
| − | proud mom ;)
| |
| − | | |
| − | | |
| − | For "mass" production I tried 2 methods:
| |
| − | RED: ink from bacteria that remains IN the filter
| |
| − | BLUE: ink from the bacteria that "somehow" passed through the filter.
| |
| − | | |
| − | | |
| − | RED: ink from bacteria that remains IN the filter
| |
| − | BLUE: ink from the bacteria that "somehow" passed through the filter.
| |
| − | | |
| − | RED: ink from bacteria that remains IN the filter
| |
| − | BLUE: ink from the bacteria that "somehow" passed through the filter.
| |
| − | | |
| − | | |
| − | Just in case, I keep my new colonies growing.
| |
| − | | |
| − | | |
| − | And made some more LB Medium for future cultures: B (Basic lb medium), M2 (Medium: Basic lb + 2gr. more of yest and peptone), R4 (Rich: Basic lb + 4gr. more of yest and peptone).
| |
| − | Good job ! I'll check the incubator it's strange that it doesn't wnat to increase the temperature to 37°C. However it gives the time to your bugs to grow slowly and to you to control them =) Note, the guy owning the incubator besore was using it to do smoked salmon... Dafuk... Anyways I see your sterility is good and you have little to no contagion which is great ! Keep up the good work =)
| |
| − | Everithing processed and stored in the fridge, prepared for the New Year 2015!
| |
| − | | |
| − | | |
| − | | |
| − | 05/01/2015
| |
| − | -------------------------------
| |
| − | On a commencé a faire la production en "masse" avec le gros machine pour centrifuger.
| |
| − | | |
| − | Il faut balancer le truc.
| |
| − | | |
| − | 3000 rpm et 10min apres...
| |
| − | | |
| − | Au lieu du alcohol on va essayer avec Acetone.
| |
| − | | |
| − | On va a faire une essaye de fixer le plotter de Guy HP 7550, si ca va pas il y a une alternative une autre vielle plotter de un collegue du fixme. On cherche des experts de electronique!
| |
| − | | |
| − | | |
| − |
| |
| − | Alternative au projet: 3D (justement parce que c'est plus facile de injecter l'encre avec le "extruder")
| |
| − | | |
| − | | |
| − | On attendre le "PLA pellets" transparent pour voir l'effet de coleur dans le fil de plastic qui sont chauffés a 200°C.
| |
| − | | |
| − | Confirmé pour le 3d! je attendre mes petites mini machines ;)
| |
| − | | |
| − | 17/11/2015
| |
| − | -----------------------------------
| |
| − | I made a quite good looking ink in the lb medium
| |
| − | | |
| − | | |
| − | I tried to break the molecules with ultrasounds BEFORE centrifuge.
| |
| − | After centrifuge, get rid of lb rests and adding acetone, this what it looks like. My yellow babies are super!
| |
| − | | |
| − | | |
| − | | |
| − | Tones of this things that I prepared before Christmas where not efficient enough, mini fail when deciding to use hundred minitubes.
| |
| − | | |
| − | | |
| − | So from now on, I meet Mr. Super centrifugator and mega tubes ;)
| |
| − | | |
| − | | |
| − | 30 ml of pure yellow ink (in suspension), great!
| |
| − | | |
| − | Tadaaa!
| |
| − | | |
| − | | |
| − | | |
| − | I want to get rid of the "non-ink" liquid and get a pigment pouder so I gonna put it to "dry" one nigth.
| |
| − | I expect to have something like this:
| |
| − | | |
| − | | |
| − | 18/01/2015
| |
| − | --------------------------------------------
| |
| − | Mega fail!!!
| |
| − | 35ml of ink wasted, dried, merged with the petri dish and impossible to use. (Acetone "eats" plastic).
| |
| − | | |
| − | | |
| − | Gladly this remains:
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | So, new batch comming....
| |
| − | | |
| − | | |
| − | Next week, starting with Lividum Janthinobacterium, also called apperntly Chromobacterium Violaceum founded on soil and in some animals like on Red Backed Salamander's (Plethodon Cinereus) back.
| |
| − | | |
| − | (original photo http://calphotos.berkeley.edu/cgi/img_query?enlarge=0000+0000+1104+0005)
| |
| − | | |
| − | Also gonna test some cutlefish ink for black.
| |
| − | | |
| − | | |
| − | 22.01.2015
| |
| − | --------------------------------------------------------------
| |
| − | Thanks to our Lab manager! We love our lab manager ;)
| |
| − | | |
| − | --------------------------------------------
| |
| − | Ingredients for DIY MSA:
| |
| − | 5.0 g/L enzymatic digest of casein ---> check!
| |
| − | 5.0 g/L enzymatic digest of animal tissue ----> peptone plus Buy or get crap meat parts and digest them with enzymes??
| |
| − | 1.0 g/L beef extract ----> beef extract, you could use "bouillon de boeuf" heat it, scrape the oil on the top
| |
| − | 10.0 g/L D-mannitol ---> check!
| |
| − | 75.0 g/L sodium chloride ----> This is salt rigth?
| |
| − | 0.025 g/L phenol red -----> have to order...
| |
| − | 15.0 g/L agar
| |
| − | pH 7.4 ± 0.2 at 25°C
| |
| − | ------------------------------------------------
| |
| − | Walking around the school one can find some inspiration...
| |
| − | | |
| − | ------------------------------------------------------------------
| |
| − | Building the prototipe
| |
| − | | |
| − | | |
| − | | |
| − | 23.01.2015
| |
| − | ----------------------------------------------------------------------
| |
| − | Showing the prototipe, all this material will be back on the lab soon ;)
| |
| − | | |
| − | | |
| − | | |
| − | ------------------
| |
| − | UNIGE 23.01.2014
| |
| − | -----------------
| |
| − | X.Perret, Senior Lecturer Microbiology Unit Laboratory of Microbial Genetics Sciences III – University of Geneva has accepted to collaborate in the project. We are working in the lab at UM under his supervision and the assistance of his students and Phd students.
| |
| − | | |
| − | | |
| − | | |
| − | 26/01/2015
| |
| − | ------------------------------------------------------------------
| |
| − | Nice! J.Lividum are growing nicely on a poor medium and scarily growing in poor TY + 1%Glycerol (86%). Although nicer color is showing with poor TY.
| |
| − | | |
| − | As in TY is growing fast enough and very nicely, we decided to continue the liquid medium with this configuration.
| |
| − | | |
| − | | |
| − | We put them 24h. on the shaker at 170rpm and 27°C.
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | 27/01/2015
| |
| − | --------------------------------------------------------------------------------------------------
| |
| − | Après 24 heures a 27°C et 170 rpm on peut dire que on voit pas le violacein dans la bouteille. Donc on va essayer de ajouter Glycerol 1% et voir comment ils se comportent.
| |
| − | | |
| − | | |
| − | 05/02/15
| |
| − | -------------------------------------------------------------
| |
| − | First Extraction following the article Pantenalla-F_2007:
| |
| − | Abstract
| |
| − | | |
| − | Aims: To analyse the environmental stimuli modulating violacein and biofilm
| |
| − | | |
| − | production in Janthinobacterium lividum.
| |
| − | | |
| − | Methods and Results: Violacein and biofilm production by J. lividum
| |
| − | | |
| − | DSM1522T was assayed in different growth conditions. Our data suggest that
| |
| − | | |
| − | violacein and biofilm production is controlled by the carbon source, being
| |
| − | | |
| − | inhibited by glucose and enhanced by glycerol. J. lividum produced violacein
| |
| − | | |
| − | also in the presence of different sub-inhibitory concentrations of ampicillin. As
| |
| − | | |
| − | opposite, the production of N-acylhomoserine lactone(s), quorum sensing
| |
| − | | |
| − | regulators was shown to be positively regulated by glucose. Moreover, violaceinproducing
| |
| − | | |
| − | cultures of J. lividum showed higher CFU counts than violaceinnonproducing
| |
| − | | |
| − | ones.
| |
| − | | |
| − | | |
| − | | |
| − | Extraction:
| |
| − | Violacein extraction
| |
| − | J. lividum was grown in 2 ml of LB or LB-U or LB-Y in
| |
| − | 24-well plates. At regular time intervals, bacterial cells
| |
| − | from a single well were harvested by centrifugation
| |
| − | (16 000 g for 20 min), and treated for violacein extraction.
| |
| − | Violacein extraction was carried out according to
| |
| − | previously described methods (Blosser and Gray 2000;
| |
| − | Matz et al. 2004). Briefly, bacterial cells were lysed with
| |
| − | 10% sodium dodecyl sulfate. After 5 min at room temperature,
| |
| − | water-saturated butanol was added (1 : 2 v/v)
| |
| − | and vortexed. After centrifugation (16 000 g for 10 min)
| |
| − | the upper phase, containing violacein, was collected. The
| |
| − | extracted violacein was quantified using a spectrophotometer
| |
| − | (OD585). The extinction coefficient of violacein
| |
| − | (0Æ05601 ml lg)1 cm)1) was used for quantitative calculations
| |
| − | (Mendes et al. 2001).
| |
| − | | |
| − | Note: We made the culture on TY and TY + Glycerol
| |
| − | | |
| − | | |
| − | 0
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | 06/02/15
| |
| − | ------------------------------------------------------
| |
| − | Red Hunting: We saw something interesting (nice reddish color) on the source's petri from CLÉMENT DRÉVO , we isolated and grow it with the assistance of Yann Pierson. Tried to grow it in LB and MSA.
| |
| − | | |
| − | | |
| − | | |
| − | But.... didn't worked so far ;) Next time
| |
| − | | |
| − | | |
| | | | |
| | SOME CONCLUSIONS: | | SOME CONCLUSIONS: |
| | TY and TY + Gly is perfect for J.Lividum (Violet) | | TY and TY + Gly is perfect for J.Lividum (Violet) |
| | MSA, TY not working for M.luteus (Yellow) | | MSA, TY not working for M.luteus (Yellow) |
| − | LB + 4gr. plus of "food" (yeast and peptone) neither. MLuteus grow better on LB agar and liquid medium at 37°C | + | LB + 4gr. plus of "food" (yeast and peptone) neither. MLuteus grow better on LB agar and liquid medium at 37°C (maybe not well calibrated machine...) |
| | | | |
| − | 09/02/15
| + | Extraction: |
| − | ---------------------------------------------------------
| + | Violacein extraction |
| − | Time to go hunting!
| + | J. lividum was grown in 2 ml of LB or LB-U or LB-Y in 24-well plates. At regular time intervals, bacterial cells from a single well were harvested by centrifugation (16 000 g for 20 min), and treated for violacein extraction. Violacein extraction was carried out according to previously described methods (Blosser and Gray 2000; Matz et al. 2004). Briefly, bacterial cells were lysed with 10% sodium dodecyl sulfate. After 5 min at room temperature, water-saturated butanol was added (1 : 2 v/v) and vortexed. After centrifugation (16 000 g for 10 min) the upper phase, containing violacein, was collected. The extracted violacein was quantified using a spectrophotometer (OD585). The extinction coefficient of violacein (0Æ05601 ml lg)1 cm)1) was used for quantitative calculations (Mendes et al. 2001). |
| − | Today we had an interesting visit to the Lab, I found 3 nice Senior women close to my desk, they suggested me to try cochenille for the red pigment et.... voilà! It's used for Red Lipsticks and so easy to find it in our daily live, garden and supermarket!
| + | ************************************ |
| − | | |
| − | http://www.sciencepresse.qc.ca/sites/www.sciencepresse.qc.ca/files/image/2011/09/image_17.png
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | 10/02/15
| |
| − | ----------------------------------------------------------
| |
| − | Building the FilaBot DIY Kit, an extruder for plastics. Easy...
| |
| − | | |
| − | | |
| − | | |
| − | 11/02/15
| |
| − | ------------------------------------------------
| |
| − | Getting there...
| |
| − | | |
| − | I love the composition of this picture!
| |
| − | Thanks ;)
| |
| − | | |
| − | 12/02/15
| |
| − | ------------------------------------------------------
| |
| − | First attempts with Filabot extruder! I've put the natural white pellets on the machine and verted the bio+ink.
| |
| − | I have some problems with the porosity of the plastics, gonna try mabe ask an expert to know how to solve this.
| |
| − | | |
| − | | |
| − | The problem seems to be solved almost, if the pigments are added BEFORE putting them into the extruder nad let them dry for a around 30min at room T°.
| |
| − | | |
| − | | |
| − | Niiiiice tryouts.
| |
| − | | |
| − | The result:
| |
| − | | |
| − | We could start printing with our Pr(ink)t as soon as I get to assembly and connect it!
| |
| − | | |
| − | I will try also injecting the living bacteria directly on the plastic and see what happens.
| |
| − | | |
| − | | |
| − | | |
| − | 17/02/15
| |
| − | ----------------------------------------------------
| |
| − | New extraction of violacein is completed in UNIGE. I brougth bacl home 6 ml of beatiful purple ink ;) Gonna set up everything for the plastice coloration and extrution.So, as I experienced before, to much liquid (ethanol, buthanol...) is forming bubles in the extruder when evaporated, trapped in the noozle and released within the warm plastic wire. So, I will try to evaporate it in a petri before extruding it, leaving it open few hours till the buthanol is almost gone.
| |
| − | | |
| − | | |
| − | Starting to build the 3D printer for the final step and see what the result looks like after printing and to check if the ink lasts 2 melting proceses.
| |
| − | | |
| − | 31/03/2015
| |
| − | ----------------------------------------------------
| |
| − | Starting engines for the exhibition:
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | 22.04.15
| |
| − | -------------------------------------
| |
| − | After Milan exposition, I'm glad to share & post some articles and press quiotes about the project: | |
| − | | |
| − | BLOG-ESPRITDESIGN.COM
| |
| − | They point out the project with the whole video about it, this one posted at the end: "Du love-hôtel pour pigeon à l’extraction de pigments de salamander pour colorer les fils d’imprimante 3D, les propositions témoignent d’un nonconformism revendiqué par l’école."
| |
| − | http://www.blog-espritdesign.com/actus/evenement/milan-2015-the-animal-party-exposition-de-la-head-31477
| |
| − | | |
| − | YOUTUBE-HEAD Geneve Channel
| |
| − | The video:
| |
| − | | |
| − | https://www.youtube.com/watch?v=VHzA6m8Jq3g#t=42
| |
| − | | |
| − | DEZEEN.COM
| |
| − | In the middle of the article they reference the project with a phot and short description: "A salamander's belly offers an alternative method for creating coloured ink for a 3D printer in a project called Pr[ink]t Plastic, it's Fantastic! by Vanesa Lorenzo Toquero, a first year master's student in Media Design."
| |
| − | http://www.dezeen.com/2015/04/16/the-animal-party-head-geneva-school-art-design-milan-2015-installation/
| |
| | | | |
| − | LETEMPS.CH
| |
| − | At the very end they point out: "Vanessa Lorenzo Toquero a imaginé un laboratoire domestique qui transforme en pigments pour imprimantes 3D des bactéries prélevées sur des salamandres."
| |
| − | http://www.letemps.ch/Page/Uuid/4243534c-df97-11e4-aa18-ff4de01147fa/A_Milan_appareils_à_selfies_et_bêtes_de_design
| |
| − |
| |
| − | IDEAT-China
| |
| − | Also in chinese with description and photo:
| |
| − | http://www.ideatchina.com/article/phone/?aid=66
| |
| − |
| |
| − | SSMMYYVRSS
| |
| − | And my blog and the WHOLE experiment with failures and success since the begging:
| |
| − |
| |
| − | http://ssmmyyvrss.tumblr.com/post/114689633939/pr-ink-plastic-its-fantastic
| |
| − | Prink
| |
| − |
| |
| − | Now that I will be free to experiment I have some ideas to continue in parallel, in a more poetique and experimental way.
| |
| − |
| |
| − | Greetings and hope you like it.
| |
| − |
| |
| − | Vanessa L.
| |
| | | | |
| | + | EXTRUSIONS--> biocoloured bioplastic: |
| | + | Building the FilaBot DIY Kit, an extruder for plastics. 40 hours of intense work. Thank Michael for the moral support and electronic supervision. |
| | + | Put the natural white pellets on the machine and verted the bio+ink. |
| | + | The problem seems to be solved almost, if the pigments are added BEFORE putting them into the extruder let them dry (because of the extraction they already have alcohol so they dry fast) for a around 30min at room T°. |
| | <br> | | <br> |
| | | | |
| − | = Grow = | + | = Results = |
| | <br> | | <br> |
| | + | [[File:Filament.jpg|400px|thumb|left| Filament ]] |
| | <br> | | <br> |
| − | | + | We could start printing with our Pr(ink)t as soon as I get to assembly and connect it! |
| − | = Mix =
| + | [https://vlorenzolana.myportfolio.com/prinkt-plastic-its-fantastic RESULT OFFICIAL PAGE PRINT PLASTIC IS FANTASTIC] |
| | <br> | | <br> |
| | + | [[File:PlasticFil3.jpg|400px|thumb|left| 3 colors, filament ]] |
| | <br> | | <br> |
| − |
| |
| | = Related Links = | | = Related Links = |
| | <br> | | <br> |
| − | | + | *[http://www.cheminfo.org/Image/Process/Test_Colonies/index.html?viewURL=https%3A%2F%2Fcouch.cheminfo.org%2Fcheminfo-public%2Fd10023865c54681d7a1c9c15a247e703%2Fview.json%3Frev%3D31-4fb461b3d4bd5314c3395d904ae6eea6 Test Colonies Online FREE] |
| | * [https://hackuarium.hackpad.com/Prinkt-plastic-its-fantastic-FLxNne2LEZD hackpad for the Prinkt plastic project] | | * [https://hackuarium.hackpad.com/Prinkt-plastic-its-fantastic-FLxNne2LEZD hackpad for the Prinkt plastic project] |
| | * [http://ssmmyyvrss.tumblr.com/post/114689633939/bacteria-ink-plastic-milano-2015 Exhibition 2015 Milano fuori salone] | | * [http://ssmmyyvrss.tumblr.com/post/114689633939/bacteria-ink-plastic-milano-2015 Exhibition 2015 Milano fuori salone] |
| Line 558: |
Line 112: |
| | [[Category:Work In Progress]] | | [[Category:Work In Progress]] |
| | [[Category:Projects]] | | [[Category:Projects]] |
| | + | [[Category:Bachelor-Master-PhD Projects]] |