Difference between revisions of "Pushing the P1"
| Line 255: | Line 255: | ||
*Good old openTaq | *Good old openTaq | ||
*DNA fragment purification by gel extraction. (cheap, easy, no need of DPNI enzymes) | *DNA fragment purification by gel extraction. (cheap, easy, no need of DPNI enzymes) | ||
| − | *Ligation through modified Gibson (lysed bacterial supernatant + enzymatic complementation (Exonuclease mostly), To investigate further see the SLiCE method) | + | *Ligation through modified Gibson (lysed bacterial supernatant + enzymatic complementation (Exonuclease mostly), To investigate further see the [Media:SLiCE.PDF SLiCE] method) |
*Transformation in chemo competent cells by heat shock (way cheaper than electro-competent cells) | *Transformation in chemo competent cells by heat shock (way cheaper than electro-competent cells) | ||
*Usual LB-agar plating with ampicilin selection | *Usual LB-agar plating with ampicilin selection | ||
Revision as of 11:38, 1 June 2016
Introduction
Having a laboratory and instruments is one thing, possessing the tools to do actual biology research is another thing. For close to two years now, the Hackuarium community has built a space, where people can work on projects related to biology among other fields. The ambition of the international DIY biology scene is to bring to the people the tools to perform and work on the whole range of biological applications, as qualitatively and cheaper than what the industrial or academic institutions do. To achieve such level, the Hackuarium lab has to be upgraded to a higher level of competency. One aspect is the P1 biosafety level where genetic manipulation opens the door to a vast range of research topic and engineering opportunities. This scientifically enriching, yet delicate step, is now in motion but in order to start practicing several items involving legal, technical and community topics have to be settled. Indeed, our laboratory practices and transparency as a citizen lab have to be absolutely irreproachable. The challenges we face are described in this document.
We will prepare for the P1 work assuming it will start out in bacteria, and then depending on future demands, we imagine adding other organisms.
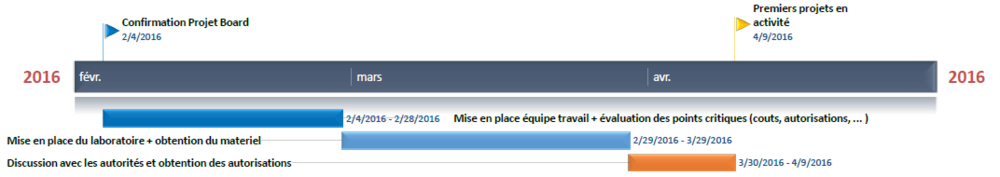
Timeline
Here is the basic timeline we will try to keep in order to make the project move on. It is obviously a "best case scenario" approximation.
The schedule could not be held due to renovations in the building
Critical Points
What we need to sort out before we start:
Announce the first activities
- YES, Luc has a form
Will the enclosure be satisfactory?
- YES, but it may be a good idea to inform the Canton
Do we need a bio safety officer? Does this person need a specific training?
- Legally yes! I think it is good if 2-3 people get this training, either at EPFL, or in Bern.
Risk assessment
- The biological risk assessment will be made through the form each member as to fill when he creates a new genetically modified strain to ensure no pathological or harmful gene is inserted in the micro-organism.
- Chemically, any inflammable liquid will be stored in the solvent cabinet. The acids and bases will be separated in two "geographically" remote cabinets. The bottles will be stored close to ground level in a resistant container able to contain the full volume of the bottles it contains to avoid spillage.
Waste elimination
- -> OK (see below)
Federal Coordination Centre for Biotechnology (where you have to notify level 1 activities with GMOs):
http://www.bafu.admin.ch/biotechnologie/01744/01745/index.html?lang=en
BSO-Curriculum (courses for biosafety officers):
http://www.bafu.admin.ch/biotechnologie/01744/02964/index.html?lang=en
Cantonal authority (Vaud, contact persons)
-Isabelle Dessaux (isabelle.dessaux@vd.ch )
-Olivier Gianina (olivier.gianina@vd.ch )
Our (very helpful) contact is:
Manuela Ocaña
Collaboratrice scientifique, PhD
Département fédéral de l'intérieur DFI
Office fédéral de la santé publique OFSP
Unité de direction Santé publique
Schwarzenburgstrasse 157, 3003 Berne
Tél. +41 58 462 63 66
Fax +41 58 462 62 33
manuela.ocana@bag.admin.ch
www.bag.admin.ch
Her initial email was as follow:
Bonjour,
L’office fédéral de la santé publique (OFSP) est, en collaboration avec l’office fédéral de l’environnement (OFEV), responsable pour les aspects de sécurité biologique en Suisse. L'ordonnance sur l'utilisation des organismes en milieu confiné (ordonnance sur l’utilisation confinée, OUC ; RS 814.912) règle l'utilisation d'organismes génétiquement modifiés, pathogènes ou exotiques en milieu confiné (par ex. laboratoires de recherche, de diagnostic ou d’enseignement et développement). Cette ordonnance a pour but de protéger l’être humain, les animaux et l'environnement des menaces et atteintes possibles résultant de l’utilisation en milieu confiné de tels organismes. Les instituts, les entreprises et les organisations qui utilisent des organismes génétiquement modifiés, pathogènes ou exotiques en milieu confiné sont ainsi tenus de notifier leur activité (classes 1 et 2) ou de demander une autorisation (classes 3 et 4).
Nous avons pris connaissance, avec grand intérêt, des informations concernant les activités et prestations proposées par votre laboratoire « Hackuarium », qui pourraient par ailleurs tomber dans le champ d’application de l’OUC. En effet, et selon le type de matériel utilisé (par ex. organismes génétiquement modifiés ou pathogènes) ainsi que la nature et l’ampleur de vos activités, ces dernières pourraient être soumises au devoir de notifier selon l’OUC. Mais avant d’entreprendre des démarches qui pourraient s’avérer finalement inutiles, je vous propose de consulter d’abord les informations disponibles sur le site du Bureau de Biotechnologie de la Confédération puis de reprendre contact avec nos services afin de clarifier au mieux votre situation.
N’hésitez à me contacter en tout temps si vous avez des questions ou besoin d’informations supplémentaires.
Pivot Points
Here we develop and tackle down the most critical points of this project. The idea is to estimate if our space can accommodate such an infrastructure and if our community has the shoulders to carry the necessary responsibilities. The points have to be assessed keeping in mind the first standardized lab procedure that will be globally used by the lab (see generalized procedure).
Lab room and material
Here we discuss the size features and needed characteristics of the room we are going to use for the lab. We also describe a material list we would need to equip the lab to the minimum.
Room features
Material
Stockage:
- Congelateur -80°C, -20°C: -50, 60C freezer used for tuna transport comes in a smaller size.
- Frigo, 4°C
- Glace
Transformation:
- Heatblock statique
- Heatblock shaker
Culture:
- Static incubator (30-37°C). There are low-cost incubators for egg hatching (reptiles, chickens). Search anibis for couveuse. http://www.amazon.com/Farm-Innovators-Model-2100-Incubator/dp/B007571D2K/ref=sr_1_3?ie=UTF8&qid=1457957604&sr=8-3&keywords=egg+incubator http://www.anibis.ch/it/animali-e-accessori-accessori-per-rettili-attrezzatrure-speciali--1007/couveuse-%C3%A0-vendre--13517987.aspx?fts=couveuse&sf=ftw&so=d&fcid=0
- Shaking incubator (reaching cold temperatures: 16-37°C). We could use a static incubator + magnetic stirrer. For low temperature, a wine cooler cellar can be found at low prices. http://www.anibis.ch/fr/vins-gastronomie-accessoires-%C3%A0-vin-etag%C3%A8res-,-armoires-%C3%A0-vins--3076/petite-cave-%C3%A0-vin--14278323.aspx?fts=cave+vin&sf=dpo&so=d&fcid=854
Post-culture:
- Centrifuge large volume
- Ultra centrifuge
- Sonication
- Centrifuge 1.5ml
- Autoclave destruction
Mobilier:
- Benches
- Armoires
Autres:
- Bec Bunzen
- Sturdy trash box
- Safe liquid waste flask
Waste eliminations
Here we describe who can and accepts to eliminate our wastes at what cost and try to estimate the frequency of elimination. We base the frequency on a per project base. How many liters of cultures are produced per production? How many biowaste bag can be filled in a week? etc...
Procedure
Based on: http://www.cusstr.ch/repository/138.pdf
- Les souches non pathogènes et non modifiées génétiquement ne nécessitent pas d’inactivation. Les déchets liquides peuvent être éliminés dans l’évier pour autant qu’il n’y ait pas d’autres contaminants p.ex. chimiques ou radioactifs. Les déchets solides doivent être éliminés avec les déchets incinérables, avec les mêmes restrictions que les liquides.
- Tout matériel de laboratoire contaminé par un OGM de classe 1 doit être inactivé avant élimination pour une filière de déchet normal. Un marquage clair des sacs à déchets biologiques doit être effectué afin de pouvoir identifier aisément les déchets autoclavés de ceux qui ne l’ont pas encore été. Par exemple, le sac auroclavé sera transféré dans un autre sac de couleur différente ne laissant plus apparaître le sigle biohazard et le contenu.
- Les objets tranchants ou coupants doivent être éliminés comme déchets spéciaux.
In english in a sentence Bio-waste bags have to be clearly distinguishable from other waste bags for example with the bio-hazard sign on them. Once inactivated (i.e. autoclaved) you have to package the waste in a way that it neither doesn't look nor doesn't smell disgusting or dangerous. Then you can discard it as household waste. If this is not possible, it needs to be discarded as special waste, how it is done at EPFL (white bag with red stripes).
Costs
Sterilisation bags: 36.- / 100 units @ Roth chemicals
Reusing large glass funnels: 0.-
Usage: Based on a 22ppl lab on full time work we use 4-6 bags / week (Yann P. EPFL lab)
WE can estimate our usage to ~1 bag/week i.e: ~2.-/month
Service providers
Since the bags of solid wastes can be eliminated through the classical way and the autoclaved liquids can be poured to the toilets the cost can be estimated negligible.
No need for special waste removal
Calculated base costs
Here we estimate the cost of starting the whole setup. Taking a standardized transfection method, expression method, cell line and antibiotic for the whole lab, how much would it cost to start it up? What are the most important enzymes? Are there opensource alternatives? We base the costs on the standardized procedure explained at the end of the document. The costs are for 1 expression from competent cells to protein purification
PCR
- 1 add-gene plasmid: 65$ + Transport (~50$)
- 1 Microsynth primer of 20 to 35bp (classic for Gibson assembly): 8.- to 12.-
- Need often 4 ~= 40.-
- ThermoFisher seems more expensive
- Fragment amplifications (based on 2 fragments)
- Gibson assembly:
- NEB 10 reactions : 160$ (That's a no)
- Home cocktail using Phusion, Exonuclease, Ligase: Which ? + Price
- Should try the cell lysate assembly, it should be cheap: Source ?
Cultures
- 20g/L == 50L == 100 to 200 expressions if we base ourselves on 200 to 500 ml expression volumes.
- On comparison we use roughly 5L/day on a 22 full time protein engineers laboratory. This is 100L / month == 200.- / month. We can base our usage to 10% of this if the medium is used efficiently. This makes 10L / month == 20.-/month
Wastes
Calculated above: 2.- / month
Chemicals
Summary
Softwares and Databases
Here we discuss the gene editing softwares to design plasmids, primers and gene building blocks. A total transparency has to be implemented in order for people to follow our activities. What kind of database will we use to store the ordered primers sequences? Same for the plasmids?
Softwares
Database
Providers
Here we discuss the possible service providers, their costs and if they are ready to deal with us. We canot create plasmids without primers or genes and therefore need providers. We cannot confirm the authenticity of our work without a verified plasmid sequence and therefore need sequencing services.
Primers
Microsynth : ~ 12.-/30 bp primer
Sequencing
Genes
Legal
Hackpad first exploration document : https://hackpad.com/Wanna-go-P1--I8F1GOCYTR9 (Most of the necessary infos can be found here)
As said previously our laboratory practices have to be irreproachable. We carry the "hacker" label and therefore, for the public opinion and the built of trust, cannot fuck around. We need to study in depth several aspects of the legislation governing genetic manipulation.
WHO
The very good lab safety manual right here
Go through it if you have the time or are curious, safety in the lab is everybody's job.
Swiss legislation
On Feb 3 2016, we got some pointers from the Vice Dean of the School of Biology of UniL who passed us information from Audvion.ch (Ingénieur de sécurité MSST)
From: Objet: AVP réponse biosécurité Re: Laboratoires P1 Date: 3 février 2016 13:58:56 UTC+1
Pour toutes les questions de sécurité biologique (biosécurité) les règles de base sont données dans deux ordonnances doit :
- OUC Ordonnance sur l'utilisation des organismes en milieu confiné https://www.admin.ch/opc/fr/classified-compilation/20100803/index.html
- OPTM Ordonnance sur la protection des travailleurs contre les risques liés aux microorganismes https://www.admin.ch/opc/fr/classified-compilation/19994946/index.html
Vous pouvez aussi consulter
- Le site de la CUSSTR (Commission universitaire pour la santé et la sécurité au travail romande) http://cusstr.ch/fr/doc/technique/detail/?idcat=14
- Un petit aide mémoire de la SUVA sur le sujet (missing link)
- Vous référer au coordinateur de biosécurité BSO de votre département
On Feb 25 2016, we got some more pointers from the Office fédéral de la santé publique OFSP
L’office fédéral de la santé publique (OFSP) est, en collaboration avec l’office fédéral de l’environnement (OFEV), responsable pour les aspects de sécurité biologique en Suisse. L'ordonnance sur l'utilisation des organismes en milieu confiné (ordonnance sur l’utilisation confinée, OUC ; RS 814.912) règle l'utilisation d'organismes génétiquement modifiés, pathogènes ou exotiques en milieu confiné (par ex. laboratoires de recherche, de diagnostic ou d’enseignement et développement). Cette ordonnance a pour but de protéger l’être humain, les animaux et l'environnement des menaces et atteintes possibles résultant de l’utilisation en milieu confiné de tels organismes. Les instituts, les entreprises et les organisations qui utilisent des organismes génétiquement modifiés, pathogènes ou exotiques en milieu confiné sont ainsi tenus de notifier leur activité (classes 1 et 2) ou de demander une autorisation (classes 3 et 4).
Nous avons pris connaissance, avec grand intérêt, des informations concernant les activités et prestations proposées par votre laboratoire « Hackuarium », qui pourraient par ailleurs tomber dans le champ d’application de l’OUC. En effet, et selon le type de matériel utilisé (par ex. organismes génétiquement modifiés ou pathogènes) ainsi que la nature et l’ampleur de vos activités, ces dernières pourraient être soumises au devoir de notifier selon l’OUC. Mais avant d’entreprendre des démarches qui pourraient s’avérer finalement inutiles, je vous propose de consulter d’abord les informations disponibles sur le site du Bureau de Biotechnologie de la Confédération (http://www.bafu.admin.ch/biotechnologie/01744/01745/index.html?lang=fr ) puis de reprendre contact avec nos services afin de clarifier au mieux votre situation.
BioSafety Officier (BSO)
Courses : http://www.bafu.admin.ch/biotechnologie/01744/02964/index.html?lang=f
Infos:
BSO, niveau de sécurité 1 (BSL1_2016)
Date: 9 septembre 2016 (1 jour)
Lieu: Université de Berne
Inscription d'ici au 30 juin 2016 à: registration@curriculum-biosafety.ch
Coûts: 550 francs par personne
Langue: anglais
Annonce confédération
Through the BAFU portail
Norms
Generalized procedure
These procedure will be implemented in the lab as first benchmark in order to have a work basis.
The "project request" procedure will be a way to ensure complete transparency and applicability. It will have to be filled before any project can start by any member willing to perform GMO activities.
They are optimized for cost & material efficiency.
Project Request
Develop a document/wiki page as template where people will have to explain the GMO they want to produce.
As an idea it could be a form or a wiki page that anybody could see structured as the following
- Goal:
- - What is the purpose of the GMO / protein you want to create OR: What question should it help you answer
- ex: - Sense endocrine disruptors / Produce methane / Produce dyes
- - What is the purpose of the GMO / protein you want to create OR: What question should it help you answer
- How does it help reach your goal
- ex: - This enzyme is known to biochemically produce red dye (add source)
- ex: - The combination of protein A,B&C could produce a sensor...
- Plasmid
- - Tell which plasmid you will use (for archive purpose)
- - Give the code of the cassette you will use
- - Give the peptide translation of such cassette
- Template
- - If you want to use several building blocks for you proteins, give details on the templates
- Primers
- - Give the primers you are going to use to realise your clones
» Project request form with above points is now here.
Bacterial
All of these components have to be open source.
Strain: e.coli
Plasmid:
Resistance: Ampicilin
DNA Amplification / Cloning
- Good old openTaq
- DNA fragment purification by gel extraction. (cheap, easy, no need of DPNI enzymes)
- Ligation through modified Gibson (lysed bacterial supernatant + enzymatic complementation (Exonuclease mostly), To investigate further see the [Media:SLiCE.PDF SLiCE] method)
- Transformation in chemo competent cells by heat shock (way cheaper than electro-competent cells)
- Usual LB-agar plating with ampicilin selection
- How do we purify the DNA from the cultures ? (yeah of course we can use qiagen minipreps but it's expensive...) There is an easy spermidine DNA prep that works all the times and costs nothing.
Primers production
Can we inspire ourselves from the cheap DIY peptide synthetiser and turn it to a primer systhetiser ? Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4045704/#!po=52.8409
Cultures
- e.coli chemo competent cells will be stored in a -80°C fridge.
- Transformation will be made by heat shock as described in ("doc name here").
- Classical LB-Amp plating with static overnight 37°C incubation.
- Liquid cultures can be either made as:
- LB-Amp followed by IPTG induction.
- (-) IPTG is expensive
- (-) Monitoring of optical density requires time + UV-spectro
- (-) Large volumes (1L medium in 5L flasks) for decent yields + large capacity incubator cooling to 16°C = more waste
- (-) Have to centrifuge in 500ml flasks = more post-culture work.
- (+) yields are often good, procedure is bulletproof
- Modified auto-inducible Terrific-Broth (TB-of-doom)
- (-) more complex medium preparation (can still be made as large stock)
- (+/-) yields are good but proteins might be hard to extract
- (+) No UV monitoring ("by eye check"), after growth expression can be made at room temp.
- (+) small 100ml cultures in 500ml flasks
- (+) all post-culture fork can be made in 2x50 ml flasks (convenient)
Protein purification
- Purification can be made by single His tag purification
- We have more than 200ml of Ni-NTA resin
- The resin can be recycled easily
- Quite simple procedure (requires 3 buffers)
- (-) Single His-tag purif will not yield absolutely pure proteins
- Concentration
- problem remains to be solved
- Storage will be done at -20°C in 40% glycerol
Yeast
Yeast specialists please provide a protocol applicable here, keep in mind cost & material optimization.
Teams & Tasks
- RFID entry (Sam)
- Asset tracking (Sam)
Milestone:
- Townhall Meeting / Ethical discussions(Luc)
- Move and build the space (Yann + everybody willing to participate)
- Standardized procedure for yeast
in the future
- Costs
- Providers - Adgene
- Softwares
- Waste handling
- Legal - FAQ, documentation wiki
- Yeast protocol
- Form