P1 Lab Activities
Our P1 lab in Ecublens was inspected by representatives of the federal office for the environment in 2019 and in 2023. More documentation of our activities can be found linked from this page.
Notre laboratoire P1 était inspecté par les mandataires de l'office fédéral de l'environnement (OFEV) en 2019 et en 2023. Plus de documentation de nos activités sont liées de cette page.
Inspiration
Several Hackuarium members had been working towards the goal of having a certified P1 laboratory since the foundation of the association in the spring of 2014.
In particular Yann P, Sam S and others did great leg-work, and even installed a 'green house' enclosure in the former open space (prior to renovation of UniverCité's second floor space). The work leading up to us finally getting the lab up and running is documented in this page.
One catalyst to get the renovated laboratory set up well was the participation of Dan Hernandez and Thomas Jordon to the "| How To Grow Almost Anything" (HTGAA) course organised by MIT professor George Church. Once we moved to Ecublens, we adapted to fit the available space.
Key Aims
To learn, have fun, and avoid problems!
History and Biosafety Officers
A P1 laboratory, allowing members to select for bacterial clones carrying plasmids they wanted to use or make, was officially functional at the old Hackuarium site in Renens between 1 November and 1 September 2018. The Hackuarium community laboratory then had to move to another location at this point.
After finding our new space in the context of a cooperative in Ecublens, we kept aiming to support members' projects and to safely pursue research, even during the time of the Covid-19 pandemic, when (not so surprisingly, after our friend Guy ran a GMO Detective workshop for our 5th anniversary festivities) we even contributed to a special 'Corona Detective' project.
One aspect that was particularly important to Hackuarium was to have trained biosafety officers to oversee the work done in the P1 laboratory.
Rachel Aronoff, who had started a P2 lab for viral work on sensory perception (in mice) at the EPFL, thus became the biosafety officer, while Olivier Emery initially agreed to take on the role of deputy biosafety officer. Later, Javier Ortiz took his place in this role.
If you need to contact them in particular about any proposed or in progress P1 activities, this is the best email address: biolab@hackuarium.ch
Many people have ore than a dozen people have already taken the "Introduction to biosafety" training necessary for full access to the P1 space. More training sessions can be organised upon request.
This page needs more updates and is still lacking its French translation. If you are interested in helping, please reach out!
P1 experiments at Hackuarium
Initially, these were all limited to bacterial growth of plasmid constructs**.
Both classical and 'synthetic biology' strategies are possible! (Please see Imre's guide for more info in regards to the latter!)
Notification to OFEV of a C. elegans crispr collaboration, to study a gene for quality control of gene expression with colleagues at Biozentrum (Basel), was additionally made in 2022.
** 'Construct' is the old school term for a plasmid, and 'to construct' is the proper verb for this process, btw, even if many people like to talk about cloning when putting bits of DNA together.
List of P1 Experiments
Make sure to link your project pages here!
All users in the P1 space are encouraged to use the on-line notebooks in Evernote, in order to help document their experiments. This method also allows ready sharing of protocols. Plasmid or other sequence information can also be readily analysed using the Benchling on-line application, and is recommended.
(more to come ! )
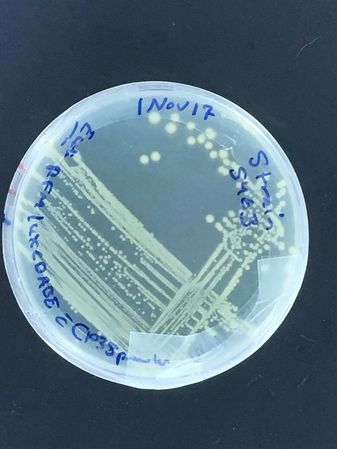
A DH5alpha test began this adventure, with first test streaks done late in 2017...
Bioluminescent strain growth (turned out our presumptive Vibrio fischeri was actually Photobacterium phosphoreum!)
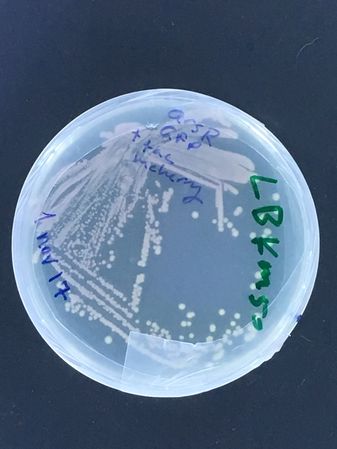
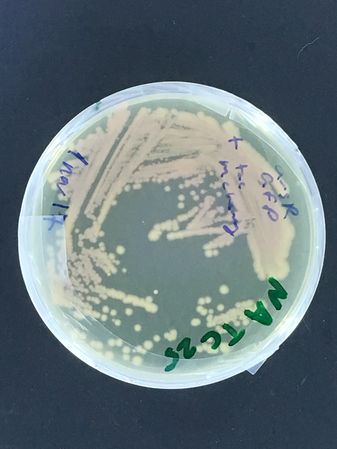
Mixed biosensor strain (containing 2 plasmids, an arsenic inducible GFP and constitutive mCherry from DMF-UNIL * see images below)
HTGAA set in the old space especially!
Open Insulin
smg-4 revisited (C. elegans gene for quality control of gene expression)
Montreux Clean Beach project water analyses, from EasyGel plates, to classic microbial media (like Levine media), to the latest card format...
SOPs and List of Potential P1 Experiments
Here is original the P1 standard operating procedure, which is still a work in progress!
Many experiments would benefit from more documentation and standard validated methods.
- sterilisation of media with the pressure cooker: we have learned that bacterial media is simpler than substrates for mycelial growth, needing more time!
- Standard Operation Procedure for inactivation of genetically modified organisms (GMOs) - bilingual FR/EN (A laminated printout of this information is found in the 'Instructions' box on the shelf in the lab.)
Validation of GMO inactivation for disposal... (done according to the above SOP using GFP+ bacteria, with good results. For details: see evernote...)
Lumina project?
more for Open Insulin?
something for your project idea?
others??
Timeline for Project Initiation
1) Talk with everyone about your ideas and what you would like to do.
2) Take the safety course and do risk assessment on your idea.
3) Start documenting your idea in the wiki. You can use this template developed by YannP as a guide.
4) Fill in the form, also developed by YannP!
5) Get going! (ask for a minigrant, if necessary for obtaining reagents or other materials.)
Gallery
The first images are of plates carrying our first plasmid strains, streaked at UNIL and grown in our own incubator, and of Vlad streaking the bioluminescent strain...
The big -80 freezer behind him can hold our stock, at DMF UNIL, until we know if the -20 method will work, or are more fully equipped!
To note: The bioluminescent strain is supposed to be moderately expressed, but on the plate after overnight incubation at 37oC, almost no light is visible by eye...
A liquid culture was also attempted next, with the same effect...
Vlad helpfully found it in the DMF collection, but more work is planned to make it bright! (Ultimately, we learned that time at 4 degrees celsius was necessary for the P. phosphoreum to produce visible light! The aldehyde substrate necessary for the light reaction, but also potentially toxic to the bugs carrying it, means that the full operon is under strict quorum regulation...)
The second strain actually carries two plasmids, a constitutive mCherry one, and an arsenic inducible GFP one... The experiment being tested is whether, by plating this strain on the two different antibiotics, new sub-strains can be selected carrying each plasmid alone... (we already have stocks of the parent strain in the UNIL freezer! It was actually made by Rachel for her 'nematode assisted biosensor analyses' from a few years back!)
There are a couple more images for the test of bacterial strain stability in the -20 freezer, with the DH5alpha strain frozen in either glycerol vs milk. This isn't strictly a P1 experiment, because these bacteria were not selected and carry no plasmids... If they are stable, however, in other words, if they can be repeatedly propagated from this stock, this will mean our P1 lab may not need (even though it would be good if we were able to get one!) a -80 freezer, as is the usual standard for strain stocks...
Come join us!
Further information
Be aware when working in the lab, and do not be in a rush!
Additionally, our basic microbiology hood is not for GMOs !
(Classic aseptic technique around a flame in the lab is always a standard part of our intro to biosafety training, but let us know if you have any questions, before initiating your experiment.)
- Here is our initial P1 standard operating procedure, which is still a work in progress!
- General Community Lab biosafety information is also available on line.
- From our pandemic experiences, here is another good starting point.
- Here is the link to all the P1 safety info, list of users and list of strains for future reference.
See something that needs mentioning? You can help contribute!
- Do you have questions? Maybe you can't reach Rachel or Javier, or want to find out more before even trying to ask them?? There is a service in the DIYBio community for this Ask a Biosafety Professional! Use it!
Remember, being clueless is no excuse!!