Micronucl
French version beta version!
A protocol for the cheek cell micronuclei tests follows:
(as all molecular microbiologists are aware, each researcher will have their particular ways to do the same things... our take on it all is that many methods may work well indeed and should be shared more!)
A basic DIT-research protocol to look for micronuclei in your own cheek cells
Note: To ideally work in pairs, at least, and score one another's cells after preparation.
This helps avoid problems of human reporting bias, it is believed.
Protocol:
- rinse mouth well with water
- use toothbrush to gently collect cells from inner cheek surface
- wash off toothbrush into little beaker with about 10ml of saline solution
- pour into 15ml conical to allow largest clumps to settle away and
- pellet cells from best part of liquid into two microcentrifuge tubes
- spin gently (no more than 5k rpm ) for 3min
- pour off supernatants, and repeat until all cells are collected
- pool pellets from the two tubes into one tube, in about 200ul saline solution
- get cell concentration of this solution (want to aim for 2000-5000 cells per slide)
- use 10ul to count on hemocytometer (the calculation if you count several cells (X) of the size of the main square = (X*#cells/X) per main square x10^4/ml)
- If necessary spin again to concentrate cells again in a small volume, as, ideally, for 'smears' will put drops of less than 50-100ul, containing about 2000 cells, onto each slide.
- Make sure slides are clean and labelled before proceeding to smears.
- (To demonstrate during workshops)
- place drop about 3/4 to end of slide, then with the edge of a second clean slide move the drop back, then smear it all forward (need pics too...)
- Let each slide air dry, can place on a hot plate.
- Fix cells to slide by briefly passing over a flame (remove soot from bottom before going onto staining).
(optional fix with methanol:acetic acid 3:1)
Stain cells with Methylene Blue (0.5% aqueous, unless optional fix – then can be made up in ethanol...)
If have slide dipping containers, coplin jars, so much the better, but can also just put a drop on the slide.
Leave stain on at least 1min then rinse with drops of water, collecting blue waste liquid* in a beaker. Let sample dry and examine under a microscope at about a 200x magnification.
Quantitation is crucial – micronuclei can arise from normal processes, but an increase of 2-4/1000 to 20 or more per 1000 cheek cells could mean one should consider deleterious environmental exposures ...
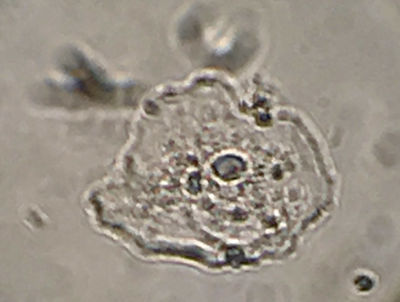
Figure 1 shows one example cheek cell containing a micronucleus, smeared, fixed and stained simply with methylene blue.
Have a look at the Prezi for the workshop done 18may19)!
Here is the 'timelapse' made during the workshop two hours in 30s??
A First Real Experiment!?
For a recent micronucleus workshop (18may19), two sisters gave their cells to us to test blind (just had samples A and B).
After counting 2 slides for each (>2000 cells), one had about 3 micronoyaux per 1000 cells and the other about 11, but it was in the opposite sense than what I predicted! (the more sporty sister actually had more…).
Anyway, both are in the ‘normal / low’ range! (basically the same given variance)
It is with more than 20 or 50 per 1000 cells that we say one should reconsider lifestyle choices (and maybe see a doctor!)!!