CBEMresults
Data from the Summer 2016 Water Quality Testing Campaign
Hammerdirt, Biodesign for the Real World, AGiR! and the Hackuarium are doing community based environmental monitoring, specifically testing water quality around Montreux Bay in Lake Geneva.
The first 'qualification' day to make sure the protocols and facilities were set to go was run on 7 June 2016.
Qualification Day
Hammerdirt volunteers met with the technical advisor from AGiR! and held a water sampling and processing 'qualification' day (Q-Day) prior to the beginning of the Water Quality Testing (WQT) pilot project.
Objectives of Q-Day
- Combine a litter survey with the collection of water samples
- Collect samples in accordance with the protocol for the WQT project
- Prepare culture medium using available laboratory equipment
- Collect samples without introducing contaminants
- Prepare samples without introducing contaminants
- Execute all tasks following laboratory safety regulations
- Verify that all laboratory equipment is operational
Meeting objectives
The success of Q-Day was based on the response to the following questions:
- Were the samples collected and plated within a 6-hour window?
- Was the litter inventory completed prior to leaving the site?
- Did field team observe proper hygiene and sampling protocols?
- Did all laboratory equipment function? Were the team members able to use equipment?
- Did the negative controls show any signs of growth?
- Did the positive controls show growth in accordance with historical results?
The survey sites for Q-Day
- Positive control Vauchère river
- Negative control : tap water
- Unknown : Bay de Vidy
Combining a litter survey with water sampling
A comprehensive litter survey can take up to two hours (depending on the size of the beach). For the Q-day exercise hammerdirt chose a small beach (Vidy) not far from an urban center (Lausanne). Two risks were immediately identified:
- Cross contamination of samples from handling litter and water sampling
- Running out of time within a 6-hour window to plate the samples
It was decided that initially the litter would be collected at the site by all team members and then, at the discretion of the team members, one individual would cease handling litter and collect the required samples.
11:50 Litter collection and inventory
12:50 Sample collection
13:40 Arrival at the laboratory
Collect samples in accordance with the protocol
The location was easy to access. The team packed soap, hand sanitizer and disposable gloves specifically for water sampling operations. The designated individual ceased handling litter and washed hands with soap and water. Clean, disposable gloves were used for the sampling process. Samples were collected by wading into the water and plunging the sampling tube roughly 50cm deep. The sampling tube was uncapped underwater and filled completely. Once the sampling tube was out of the water a portion was poured off and the sampling tube was capped and put on ice. All team members washed hands and used hand sanitizer at the end of the operation.
Prepare culture medium using available laboratory equipment
For Q-Day operations we used a dehydrated culture media, eosin methylene blue agar or Levine media produced by Oxoid (product number CM0069). The culture media that was ordered and that will be used for the WQT project, ECA Check Easygel, had not arrived by the scheduled date for this exercise. Such media requires significantly more preparation and handling than the ECA Check Easygel. The following steps needed to be completed prior to plating (within the 6-hour limit):
- Suspend 37.5g in 1 litre of distilled water.
- Bring to a boil to dissolve completely.
- Sterilise by autoclaving at 121°C for 15 minutes.
- Cool to 60°C
- Shake the medium in order to oxidise the methylene blue
- Pour medium into sterile petri dishes
- Cool medium to solid and introduce water samples
- Prepare samples without introducing contaminants
Collected samples were stored in the laboratory specimen refrigerator at 4c while the chromogenic medium was prepared. The work surface, a fume hood, was sterilized with 70% ethanol prior to pouring the chromogenic media into disposable, sterile petri dishes. The surface was sterilized again prior to plating of the water samples.
Sterile, disposable pipettes were used and changed for each water sample. Participants used disposable latex gloves and a Bunsen burner was lit during all pouring and plating operations.
Three samples from the positive control and the unknown were plated as well as one sample from the negative control (tap water).
Plated samples were covered and placed in the incubator at 37oC for 24hours.
Were the objectives met?
- Were the samples collected and plated within a 6-hour window? Yes, samples were collected at 12:50 and plated at 18:30.
- Was the litter inventory completed prior to leaving the site? Yes the results can be seen here :Vidy inventory
- Did field team observe proper hygiene and sampling protocols? Yes, field participants prepared and utilized a field hygiene kit including soap, hand sanitizer and disposable gloves. The depth of water sample was observed.
- Did all laboratory equipment function? Were the team members able to use equipment? Yes, although prior organization and identification of the proper functioning equipment could have reduced the preparation time.
- Did the negative controls show any signs of growth? No, the negative controls exhibited no growth. Indicating that no contamination occurred during sample handling and processing.
- Did the positive controls show growth in accordance with historical results? Yes, in accordance with the experience of the technical advisor.
Remarks
- Field staff will need to organize activities appropriately especially in regards to the times required to inventory litter and the 6-hour time limit for water samples.
- A bottle of water should be included in the field hygiene kit to rinse hands.
- A dedicated space needs to be attributed in the lab for this project.
- The incubator needs to be reserved ahead of time for sample processing (avoid conflicts with other projects).
- The level of the fume hood should be verified, the cooled plates showed a slight unequal distribution.
- A training session with ECA Check Easygel should be scheduled.
Conclusion
An opening day can be scheduled, hammerdirt staff are competent and all the necessary equipment is available at the lab.
Results of Microbiological Analyses
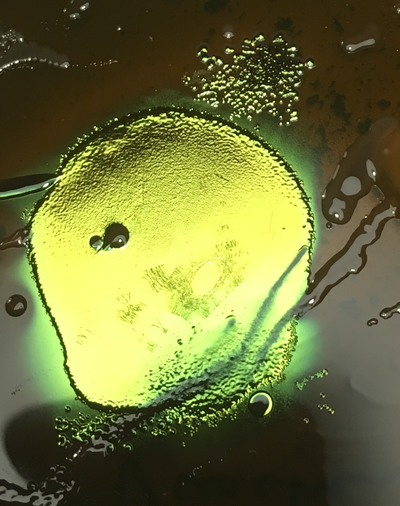
The samples were removed from the incubator after 24hours of incubation at 37°C. At least 4 colony morphologies were visible on the Levine media plates, including metallic green E. coli and various purple and pink colonies, and scored.
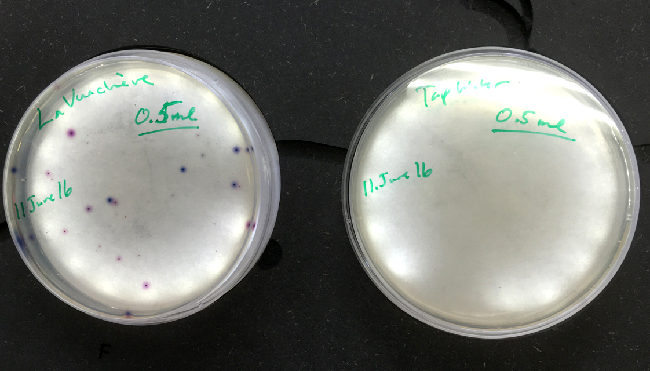
There were no visible colony forming units in the tap water or the sample taken from Vidy beach. The number colony forming units of E.coli counted from the Vauchère samples are presented in the table below.
Colony forming units counted Vauchere
| Counts | Vauchere 1 | Vauchere 2 | Vauchere 3 | Avg | Std Dev |
|---|---|---|---|---|---|
| AGiR! counts | 7 | 3 | 29 | 13 | 14 |
| Hammerdirt counts | 7 | 3 | 27 | 12 | 13 |
The ECA Check EasyGel material (pre-treated plates and individual liquid media bottles) arrived on June 11th. A control of 0.5ml of the same tap Water and Vauchère (saved in the refrigerator) samples were plated. With the EasyGel plates, it is much simpler to score the colony forming units than on the Levine media plates. Thank you, Hackuarium, for the microgrant!
To go back to the initial project page